What Best Describes Cytokine Release Syndrome Crs
Immune effector cell-associated neurotoxicity syndrome ICANS is a. While CAR-associated Hemophagocytic Lymphohistiocytosis-like syndrome CarHLH manifestations may overlap with CRS it has also been described as a.
Commonly referred to as an infusion reaction it results from the release of cytokines from cells targeted by the antibody as well as immune effector cells recruited to the area.

. The onset and peak of Cytokine Release Syndrome CRS generally occurs in the first week after CART infusion and precedes the onset of ICANS Immune Effector Cell-associated Neurotoxicity Syndrome. Cytokine Release Syndrome CRS. Cytokine release syndrome CRS is a life threatening toxicity associated numerous immunotherapeutic techniques involving monoclonal antibodies bispecific antibodies and adoptive T cell therapies.
Cytokine-release syndrome is a symptom complex associated with the use of many monoclonal antibodies. These new treatments are associated with rare toxicities such as cytokine release syndrome CRS defined by fever tachypnea headache tachycardia hypotension skin rash andor hypoxia. Describe cytokine release syndrome CRS and neurotoxicity associated with CAR T-cell administration Identify currently approved CAR T-cell products and their place in therapy Identify appropriate treatment strategies for CRS and neurotoxicity Nurse Describe cytokine release syndrome CRS and neurotoxicity associated with CAR T-cell administration.
7-8 days Manifestation may include. Cytokine release syndrome CRS is an acute systemic inflammatory syndrome characterized by fever and multiple organ dysfunction that is associated with chimeric antigen receptor CAR-T cell therapy therapeutic antibodies and haploidentical allogeneic transplantation. Cytokine release syndrome CRS is a CAR-T therapy-related adverse event.
Bio-Techne offers the most extensive portfolio of immunoassay platforms on the market for measuring CRS-associated inflammatory cytokines. CRS analyte One of the most prominent toxicities associated with cancer immunotherapies is cytokine-release syndrome CRS which can be fatal if not properly identified and managed. CRS was defined by the ASTCT consensus group as a supraphysiologic response following any.
CRS may be associated with cardiac hepatic andor renal dysfunction. The syndrome occurs when immune cells are activated and release large amounts of into the body. In this case we observed nivolumab-induced CRS in a patient with gastric cancer.
Atrial fibrillation and ventricular. HCRU within the dates of onset and resolution of CRS and NE was identified from case report. Serious events may include.
Chimeric antigen receptor T-cell CAR-T therapy yields durable responses in patients with relapsedrefractory diffuse large B-cell lymphoma rr DLBCL. A 43-year-old male with advanced gastric cancer was treated with nivolumab as a third-line. There are two basic mechanisms which describes development of CRS after therapy involving monoclonal antibodies mAB.
Cytokine release syndrome CRS is a systemic inflammatory response that can be triggered by a variety of factors such as infection and certain medications including monoclonal antibodies. While mild cases can present as flu-like illness more severe responses may lead to of life threatening cardiovascular pulmonary and. The symptoms can become severe quickly.
Cytokine release syndrome CRS is a potentially life-threatening systemic disease that has been observed after treatment with antibodies and adoptive T cell therapies. CRS has been described after the infusion of several antibody-based therapies including rituximab 10 11 obinutuzumab 12 alemtuzumab 13 brentuximab 14. Fever hypotension tachycardia hypoxia and chills.
Cytokine release syndrome CRS is observed with adoptive T-cell therapies ACT with varying frequency. CRS occurs when the immune system responds too. The clinical signs of CRS include fever hemodynamic instability and capillary leak which correlate with T-cell activation and elevated cytokine levels.
Recognize Symptoms Early Typical onset. Cytokine release syndrome CRS is a systemic inflammatory response that may be initiated by a variety of factors including T-cell therapies. In June 2018 in an effort to standardize CRS grading the American Society for Transplantation and Cellular Therapy ASTCT developed the ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells Tables 2 4.
Cytokine release syndrome CRS is a potentially life-threatening systemic inflammatory response observed following administration of antibodies and adoptive T. CRS is a systemic inflammatory response that occurs following an extensive release of inflammatory cytokines secondary to the activation of myeloid cells and lymphocytes and is mainly characterized by rash fever mental status changes etc which is more apparent in patients undergoing immunotherapy for malignancies especially lymphomas5. They are type 1 reaction.
CRS can be life threatening and may require steroids which hinder the effectiveness of ACT. Cytokine release syndrome CRS can cause a variety of symptoms including fever headaches and nausea. Cytokine release syndrome CRS is a systemic inflammatory response that can be triggered by a variety of factors such as infections and certain drugs causing pathologic over-activation of T cells.
When cytokines are released into the circulation systemic symptoms such as fever nausea chills. To date clinical trials of different CAR-T products have not been align. 2-3 days Typical duration.
Cytokine release syndrome CRS is a collection of symptoms that can develop as a side effect of certain types of immunotherapy especially those which involve T-cells. The physiopathology of CRS is caused by the release of effector cytokines IFNγ TNFα Interleukin 2 which are responsible for activating monocytes and macrophages. Apart from being used in the treatment of chimeric antigen receptor car t-cell-induced severe or life-threatening cytokine release syndrome crs in adults and pediatric patients 2.
However the immune activation responsible for high remission rates is also responsible for the unique treatment-related toxicity of cytokine release syndrome CRS. We assessed incidence healthcare resource utilization HCRU and costs of CRS and NE in pts in TRANSCEND receiving liso-cel an investigational anti-CD19 CAR T cell product administered as a defined composition of CD4CD8 CAR T cells.

Diagnostic Biomarkers To Differentiate Sepsis From Cytokine Release Syndrome In Critically Ill Children Sciencedirect
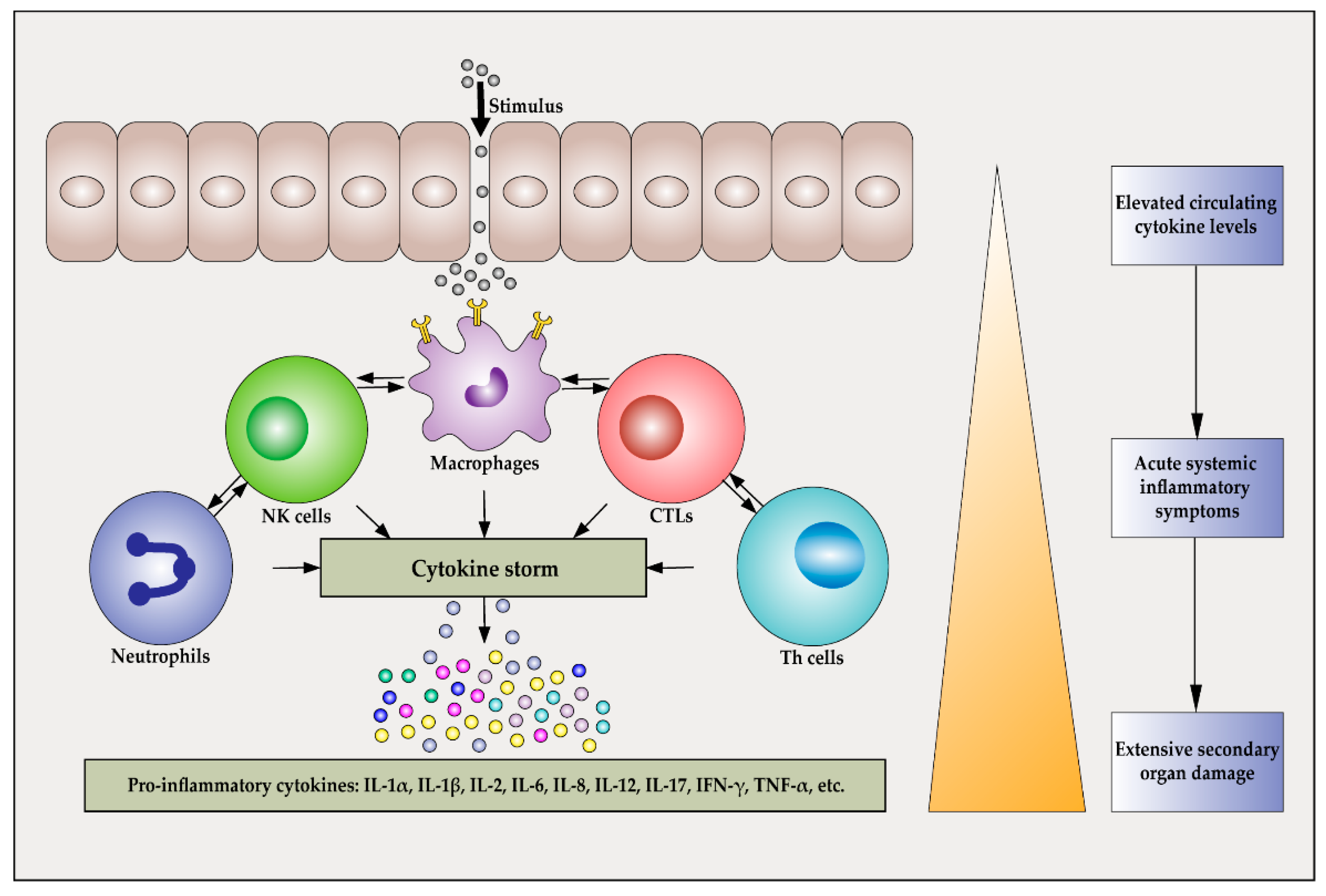
Ijms Free Full Text Pathogenesis And Treatment Of Cytokine Storm Induced By Infectious Diseases Html

Grading And Management Of Crs Considerations And Approaches For The Download Scientific Diagram
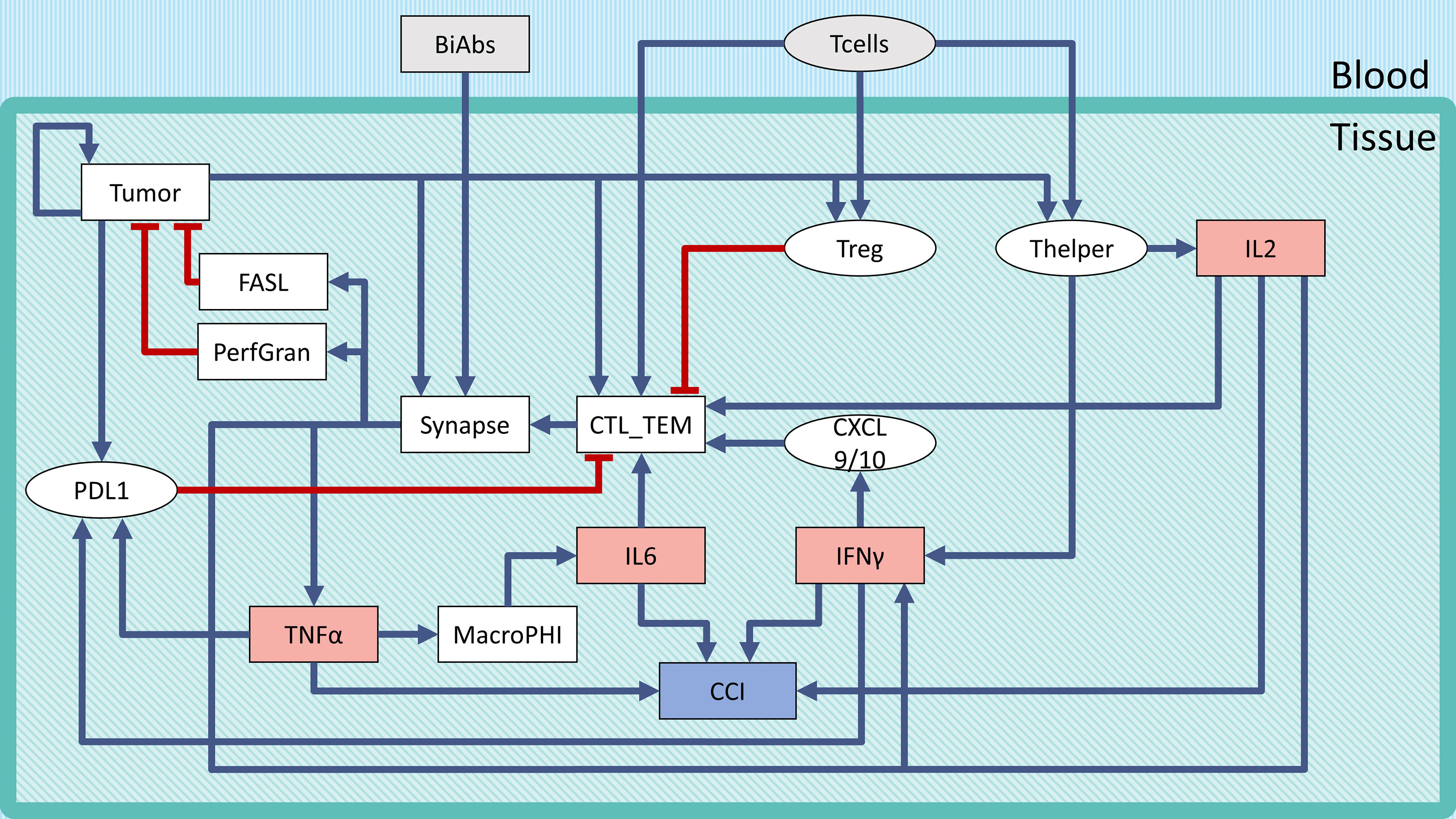
Frontiers Computational Analysis Of Cytokine Release Following Bispecific T Cell Engager Therapy Applications Of A Logic Based Model Oncology
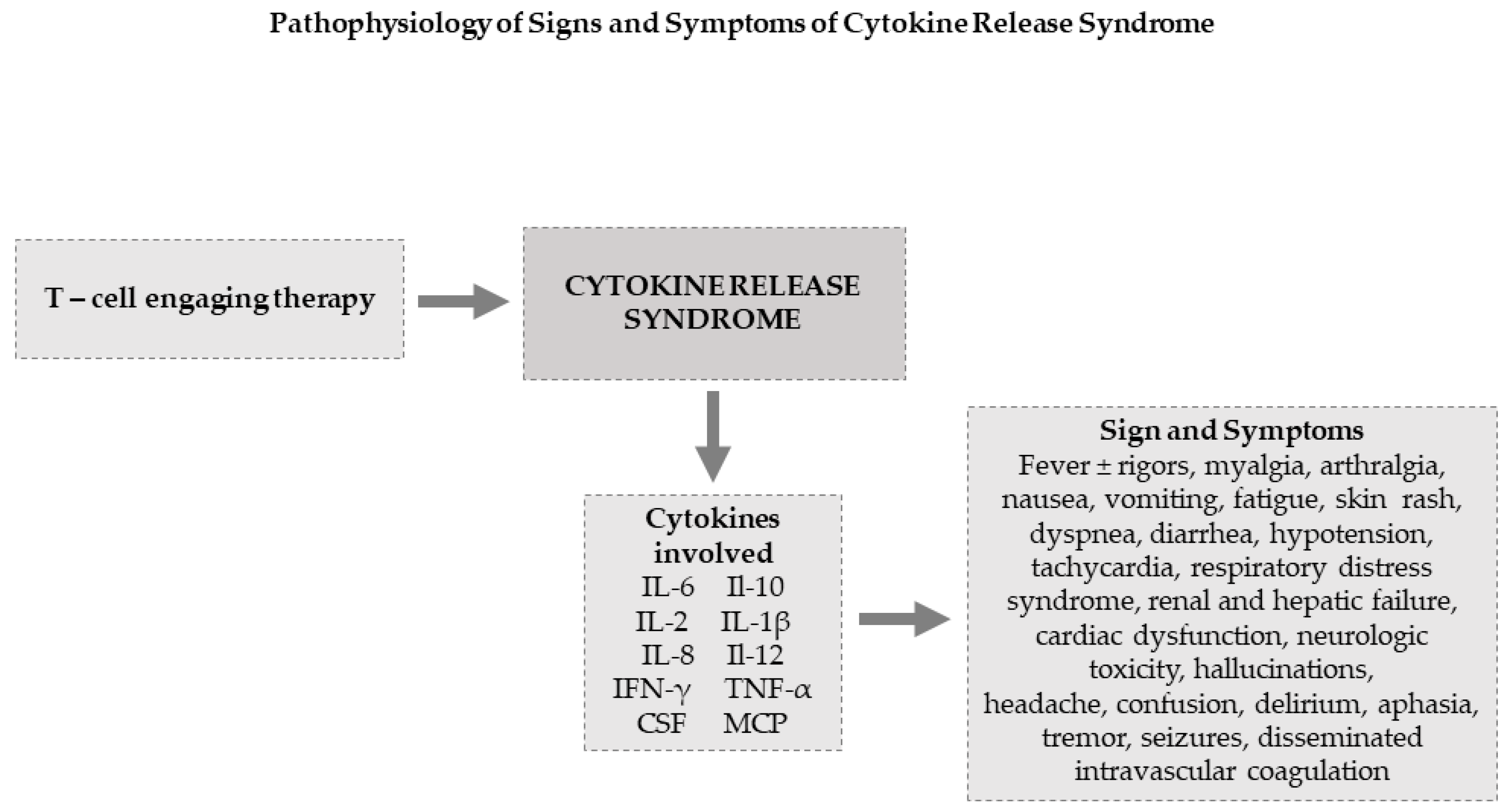
Ijms Free Full Text Cytokine Release Syndrome Associated With T Cell Based Therapies For Hematological Malignancies Pathophysiology Clinical Presentation And Treatment Html
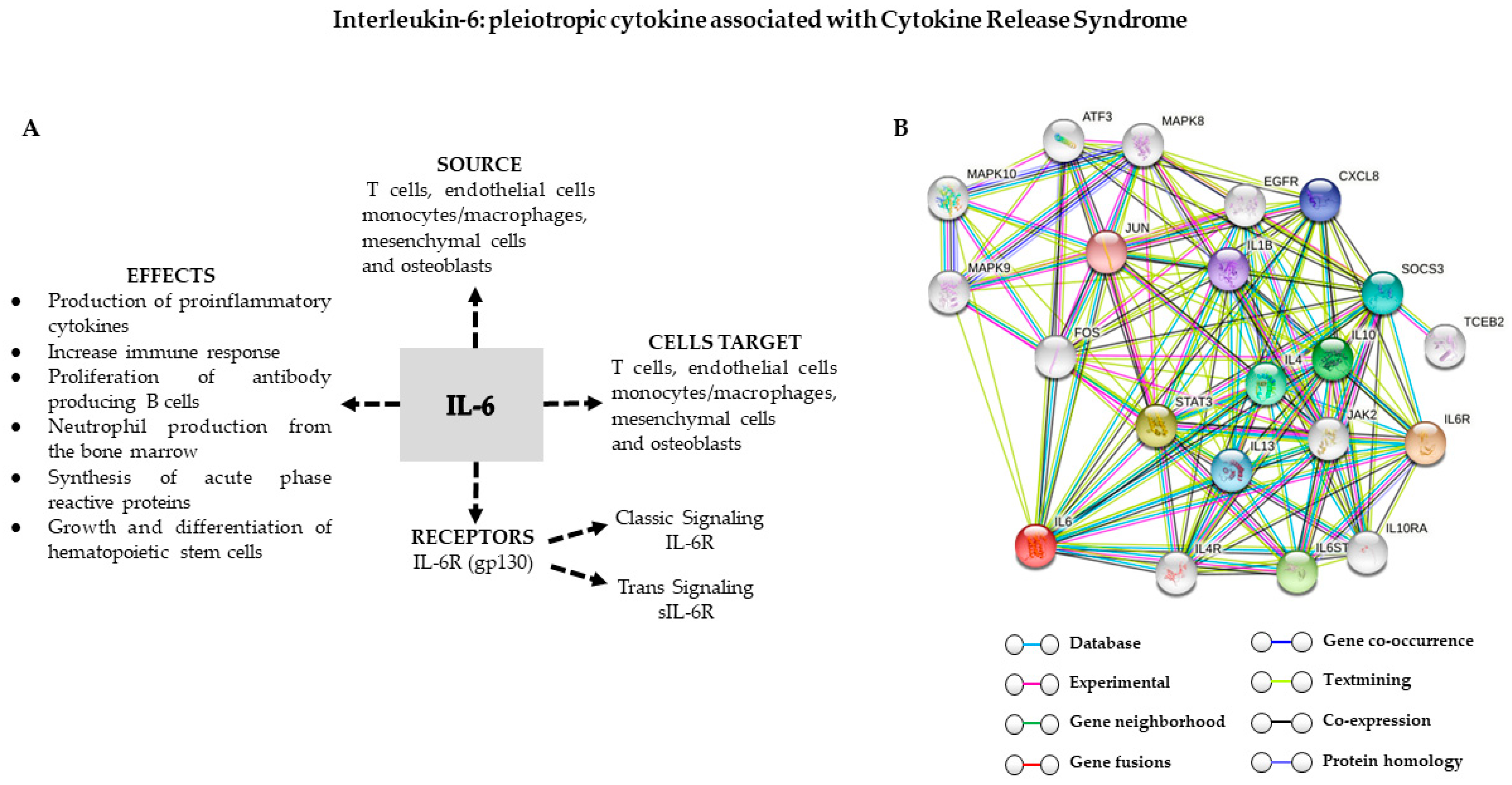
Ijms Free Full Text Cytokine Release Syndrome Associated With T Cell Based Therapies For Hematological Malignancies Pathophysiology Clinical Presentation And Treatment Html

Covid 19 And Cytokine Storm Syndrome Are There Lessons From Macrophage Activation Syndrome Translational Research
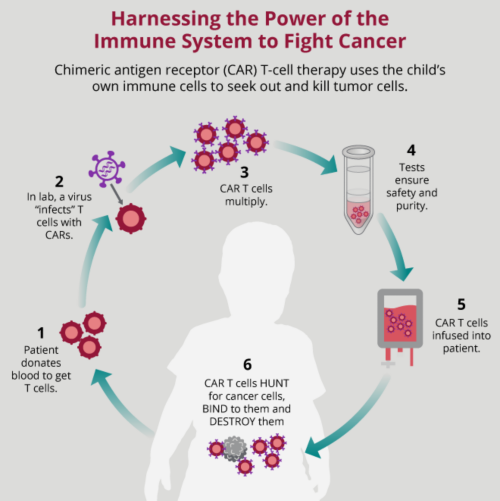
Cytokine Release Syndrome Crs After Immunotherapy Together
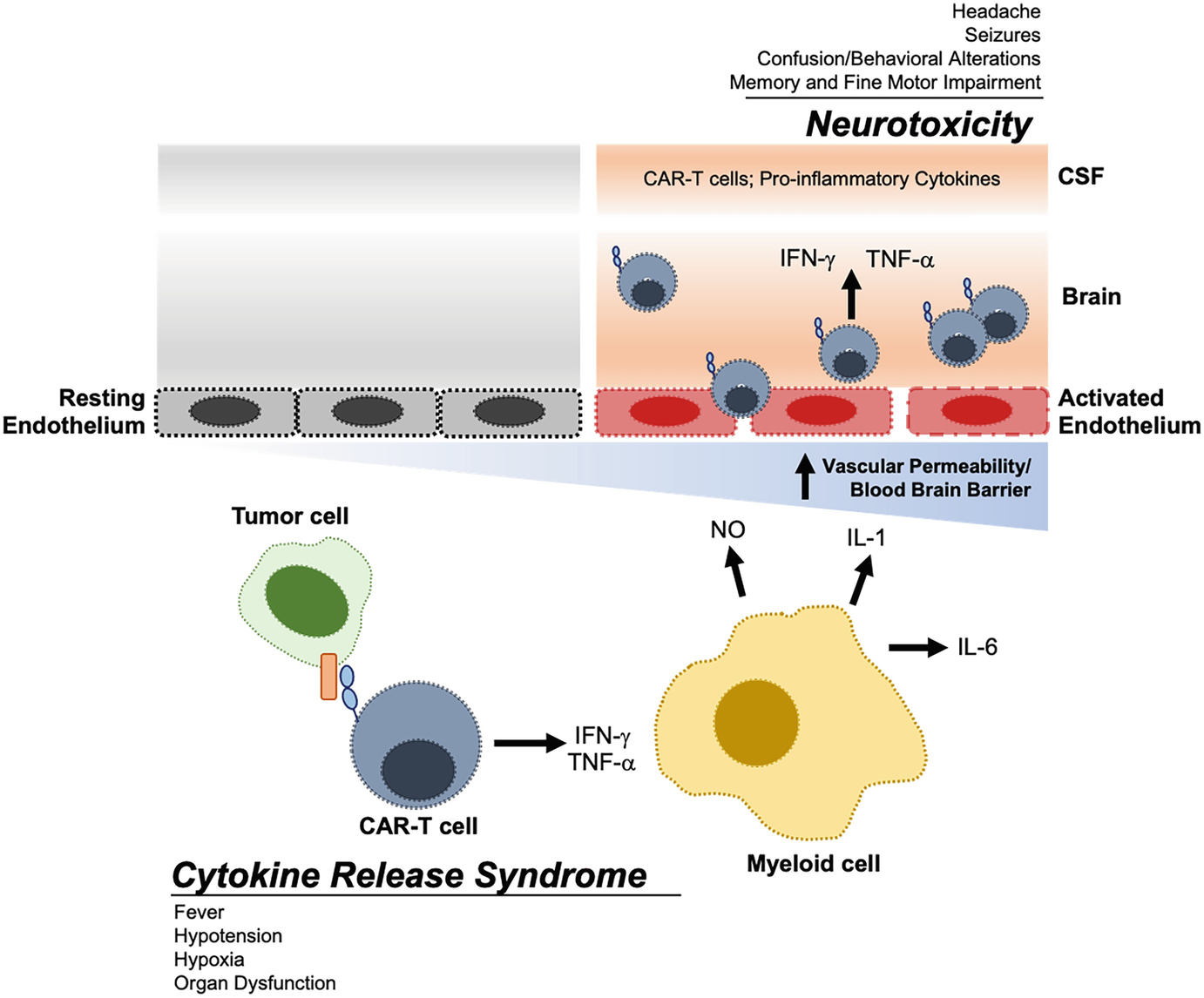
Car T Cell Complications Springerlink
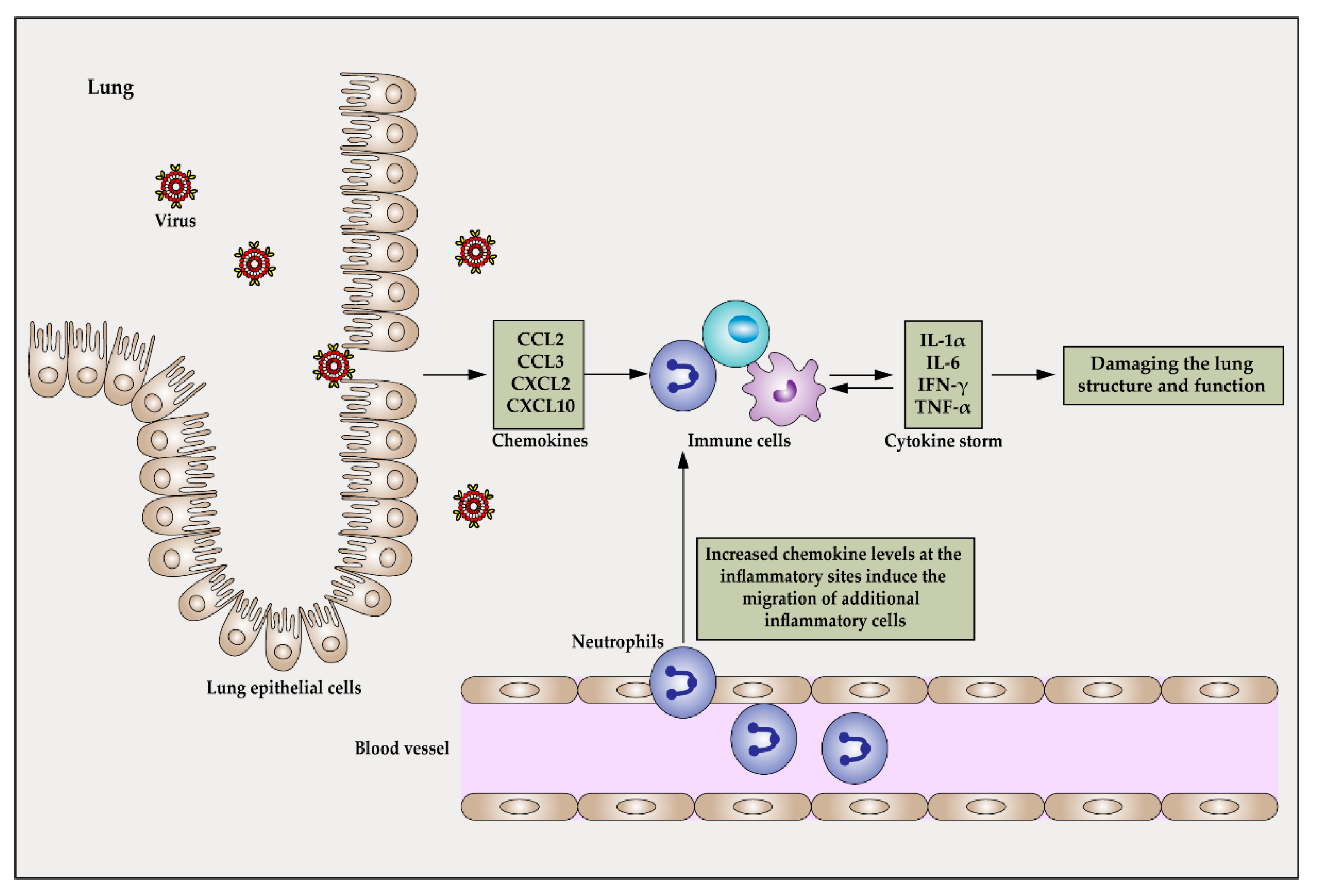
Ijms Free Full Text Pathogenesis And Treatment Of Cytokine Storm Induced By Infectious Diseases Html

Covid 19 A New Virus But A Familiar Receptor And Cytokine Release Syndrome Abstract Europe Pmc

Hypercoagulability Of Covid 19 And Neurological Complications A Review Journal Of Stroke And Cerebrovascular Diseases
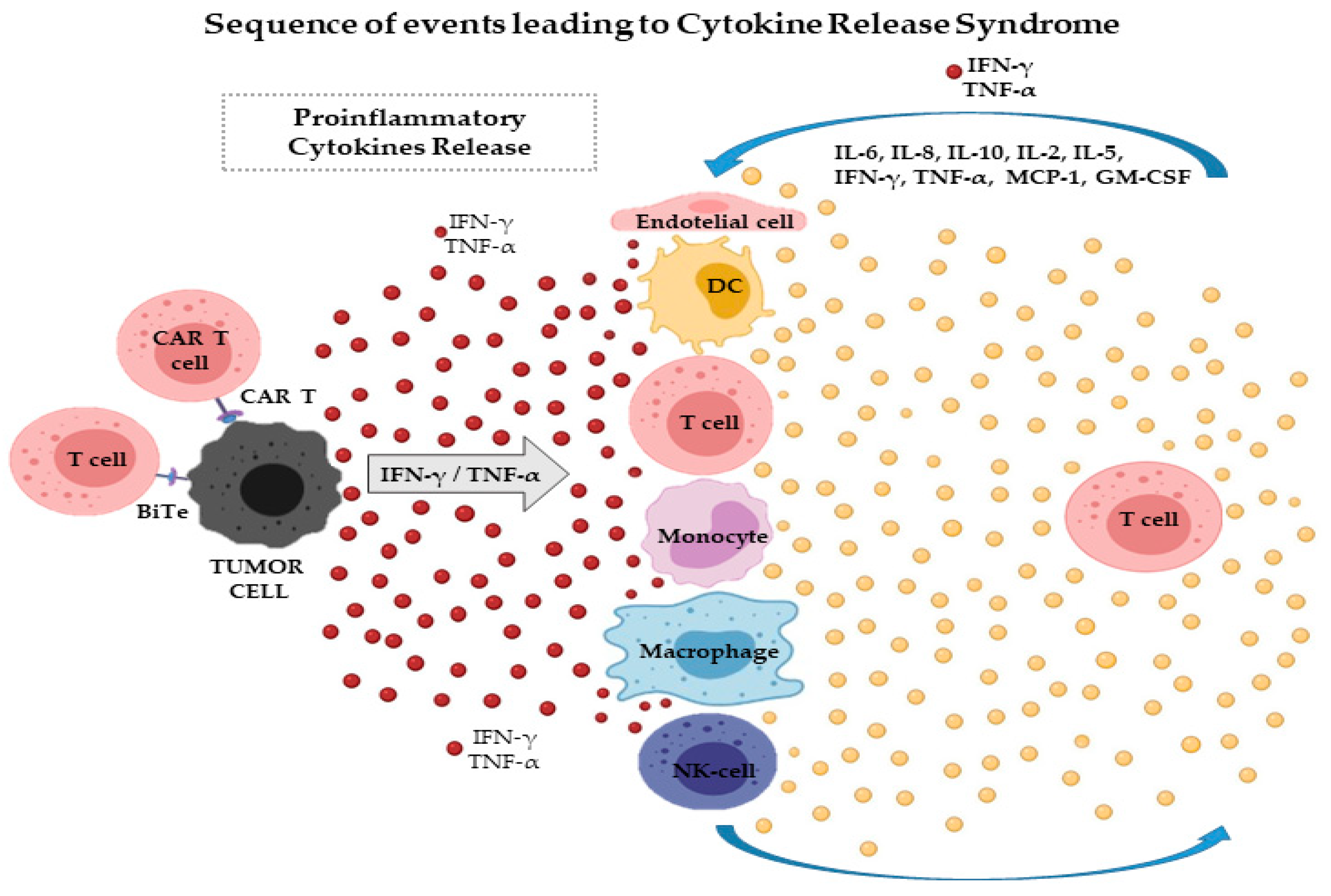
Ijms Free Full Text Cytokine Release Syndrome Associated With T Cell Based Therapies For Hematological Malignancies Pathophysiology Clinical Presentation And Treatment Html




Comments
Post a Comment